Clinical Updates


Launch of Generic Xarelto (Rivaroxaban) Tablets
Generic Xarelto (Rivaroxaban) has recently been approved by the FDA and released in 2.5 mg…


Introducing Journavx™: A New Non-Opioid Pain Relief Medication.
Vertex Pharmaceuticals has introduced Journavx™ (suzetrigine)—a non-opioid medication for moderate-to-severe acute pain in adults. Unlike…
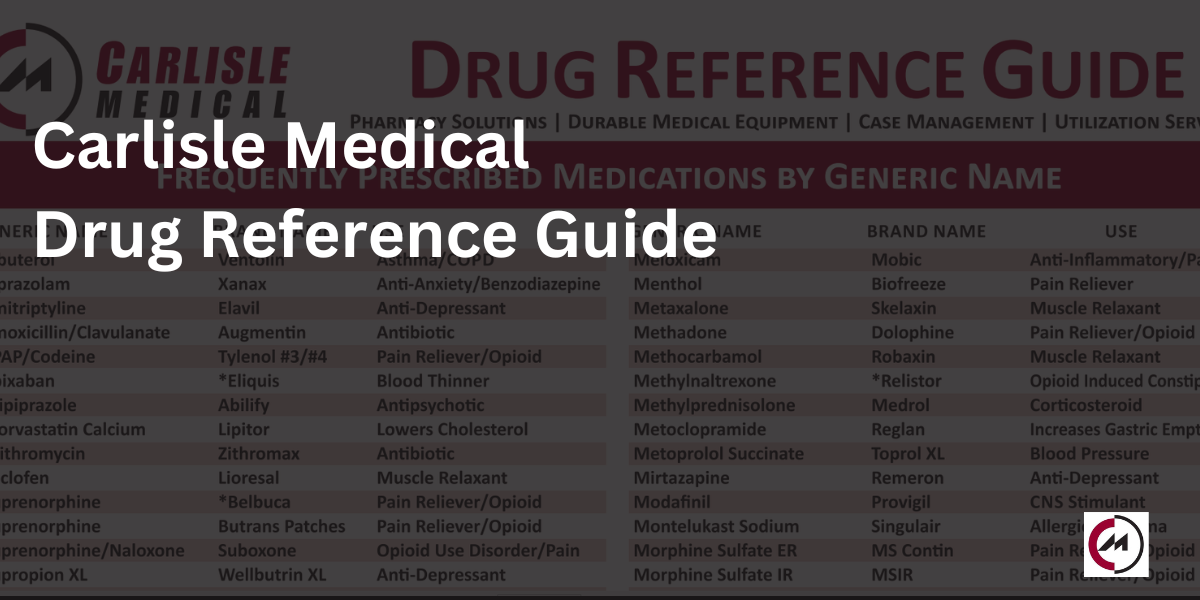


The Carlisle Drug Reference Guide
This guide provides a list of frequently prescribed medications by brand name, their generic name,…


Launch of generic Motegrity (Prucalopride) tablets
Generic Motegrity (Prucalopride) has recently been released and is approved by the FDA. Prucalopride…


Anticipated Generic Releases 2025
There are several brand name medications that will lose exclusivity in the first half of…


LSU Health New Orleans Announces Non-Addictive, Non-Toxic Painkiller
LSU Health New Orleans announces the discovery and clinical development of a new non-opioid therapeutic…


FDA approves First Nasal Spray for Treatment of Anaphylaxis (neffy) epinephrine nasal spray
Neffy (epinephrine 2 mg/dose) is a nasal spray which may be given to treat a…



The Carlisle Drug Reference Guide
As we enter 2024 we would like to remind you of the Carlisle Drug Reference…


Opvee (Nalmefene) Will Be Released in the Fourth Quarter of 2023
Opvee (Nalmefene) Will Be Released in the Fourth Quarter of 2023 Opvee is indicated for…


Release of Zavzpret In Late July 2023
Release of Zavzpret In Late July 2023 Zavzpret is indicated for the acute treatment of…


